Lipids are stored in cellular locales called “lipid droplets.” The lipid droplet surface is a lipid monolayer coated with specific proteins. The best known lipid droplet surface proteins are “PAT” (Perilipin, ADRP and TIP47) domain proteins, or “Perilipins.” Inside the lipid droplet is a core of lipids made up of triacylglycerol (TAG) and esterified cholesterol. Under states of starvation, lipases are recruited to lipid droplets to release fatty acids from TAG (see below).
Recruitment of the lipase Adipose Triglyceride Lipase (ATGL) and Hormone-Sensitive Lipase (HSL) to the lipid droplet can either be blocked or promoted by Perilipins. In mammalian cells, Perilipin 1 (PLIN1) blocks lipases from lipid droplets during basal conditions. However, during starvation, PLIN1 is modified and becomes a recruiter of HSL. As predicted by the role of PLIN1 in preventing recruitment of lipases under basal conditions, plin1 mutant mice are lean, probably because they are less able to store fat.
Mammalian cells have five Perilipins, yet Drosophila only have 2. This genomic simplicity also simplifies studies of Perilipin function. Bi et al. took advantage of Perilipin simplicity in Drosophila in their 2012 paper entitled “Opposite and redundant roles of the two Drosophila perilipins in lipid mobilization” in the Journal of Cell Science. This blog highlights the major findings from that paper.
Bi et al. found that both Drosophila PLIN1 and PLIN2 proteins localize to the surface of lipid droplets.
Flies mutant for PLIN1 have large lipid droplets (below, left two panels) and do not mobilize lipids during starvation (below, right, Bi et al., 2012). Therefore, PLIN1 promotes lipolysis during starvation.
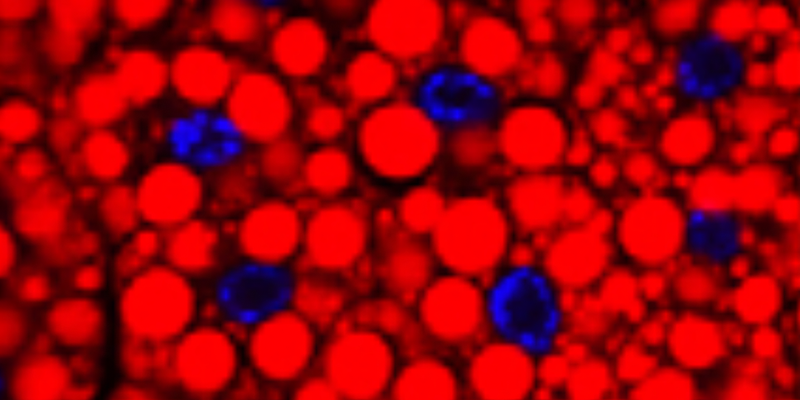
Next, Bi et al. showed that PLIN1 likely promotes lipolysis during starvation through the recruitment of the lipase HSL to lipid droplets. In wild type cells from starved animals, HSL localizes to the surface of lipid droplets (below, A). That localization doesn’t occur in plin1 mutants (below, B).
These data from Bi, et. al. indicate that PLIN1 promotes the mobilization of lipids from lipid droplets. During starvation, PLIN1 likely promotes lipid mobilization through recruiting the lipase Hsl to the lipid droplet surface.
Prior studies showed that Drosophila PLIN2 has an opposite role…PLIN2 prevents lipid mobilization by shielding lipid droplets from ATGL. Bi, et. al think PLIN2 shields lipid droplets from Hsl too, because Hsl was observed on the lipid droplet surface in plin2 mutant cells from well fed animals (data not shown). Well fed wild type larvae do not have Hsl on the surface of their lipid droplets (above, A). Below is a model depicting the roles of PLIN1 and PLIN2 in the regulation of the lipases ATGL and HSL in Drosophila lipid droplets.
However, this model is overly simplistic. Bi et al. created flies that were doubly mutant for plin1 and plin2. The model above suggests that plin1; plin2 larvae might have a phenotype equivalent to, or intermediate between, the plin1 and plin2 single mutant phenotypes. However, the plin1; plin2 larvae have smaller lipid droplets then the plin2 mutant (not shown). This implies that there are scenarios in wild type animals where PLIN1 and PLIN2 both function to prevent lipid mobilization. I suspect that the model above is mostly accurate, yet when gene dosage of PLIN1 and/or PLIN2 is altered — like in the double mutant situation — these proteins can compensate for one another.