We’ve not had a worm post in awhile, but that doesn’t mean that worms (and we) haven’t been busy. We’ve been optimizing and conducting a secondary assay on them to determine if the npc disease pathology can be resolved by testing the hits that we obtained in the primary assay. As you might remember from our earlier blog post on worms, our primary screen involved identifying chemical compounds that increased the overall growth of the worms. Compounds that were identified this way were re-tested in the same assay format to verify that the first finding held up.
Once this was done, we started working on developing a secondary assay related to NPC to confirm if the chemical compounds we identified improved a second endpoint related to NPC. With this goal in mind, we came up with an assay to measure eggs produced by the animal at the start of its reproductive maturity. We call this the Egg-in-Worm (EIW) or Egg-retention assay.
Our NPC mutant worms show slower growth and differences in brood sizes. These differences are further magnified by cholesterol deprivation. Rather than design a longer term assay to study if our ‘hits’ increased brood sizes, we decided to measure a surrogate of the same using the EIW assay. We conducted the assay optimization and the screen for this in two stages-
Stage 1: Low Throughput Assay
In the early stages of assay development, it is important to establish that the endpoint that one intends to measure is robust, reproducible and importantly represents a significant difference between disease and non-disease states. For our assay, we compared our untreated, diseased animals to cyclodextrin treated animals. Because we need to measure animals in the same stage of reproductive development, we had to obtain synchronized populations of animals to expose to the drug. To do this, we grew large quantities of worms to adulthood (gravid or egg bearing worms) on plates. These worms were washed and then bleached to obtain eggs. The eggs were then hatched out in a salt buffer like M9 buffer overnight to obtain synchronized stage L1 larvae.
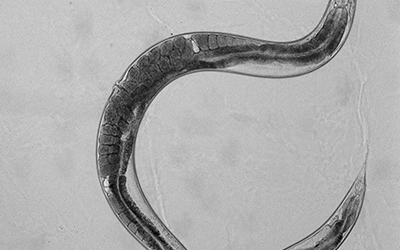
These L1 larvae were then placed on cholesterol-supplemented and cholesterol-deprived plates alongside plates that contained cyclodextrin under both conditions. Larvae were allowed to grow on these plates for 48 hours by which time they reached Day 1 of adulthood. Day 1 adults of each treatment group were picked onto an unseeded plate in turn. This allowed them to be cleared off the bacteria adhering to their cuticles. These worms were then placed into a little (2uls) droplet of M9 buffer placed on an agarose pad on a glass slide. A coverslip containing little clay feet at the corners was used to secure the worms to the pad. Worms were then viewed under a microscope at different magnifications to determine the eggs retained in them. While viewing the worms in this manner, it is important to check the image under different focal planes because fast-growing worms may have more eggs under the single row of eggs! Under these experimental conditions we found that our NPC mutant worms showed differences in the average number of eggs retained in them upon exposure to cholesterol supplementation and deprivation with and without cyclodextrin (i.e. all treatment groups). Check out these representative images of worms from each treatment group:




Stage 2: High Throughput Validation Assay
In the second stage of assay development, we tried to determine if we could convert this endpoint to an automated image readout. Instead of imaging intact worms on slides, we used a terminal bleach on synchronized, drug-exposed animals to envision and count the retained eggs. Upon exposure to bleach, the cuticle of the adult worms disintegrates within 5-10 minutes after which eggs that are released from the uterine cavity can be easily visualized. Eggs have a protective shell that prevents their rapid disintegration by bleach. However, because longer exposures can also disintegrate eggs, it was important that we find a way to either score animals quickly or terminate the bleach reaction to image the worms quickly. A second problem with this assay and using automated image quantification is that eggs tend to be released in a cluster making it difficult for an imaging program to segment them properly and quantify them. A third problem was my frequent foe- dust.
To optimize this assay and improve throughput, we incorporated a few fixes. Synchronized L1 larvae were first exposed to ‘hit’ compounds, negative control and cyclodextrin for a period of 48 hours. On Day 1 of adulthood of worms, a 384-well plate containing 50uls of 0.1% Triton X in distilled water was prepared. Worms from each treatment group were cleaned on an unseeded plate and then placed one at a time into each well of the 384-well plate. Once worms of all treatment groups were moved into the plate, a multichannel pipette was used to introduce a dilute bleach solution into each well of the plate. Fifteen minutes later the whole plate was imaged using the ImageXpress, Molecular Devices Inc. A 2X objective captured each well of the plate with enough clarity that eggs could be manually counted or counted using an automated image algorithm. While it didn’t eliminate the problem completely, the presence of Triton X, a detergent, prevented the released eggs from clumping together while preventing too many bubbles. This allowed us to capture individual eggs.
Finally, to eliminate my third foe, dust and particulate matter, we made sure we passed every solution through a 0.2micron filter to ensure that our solutions were free of floating particulate matter. The plate bottom itself was covered using a transparent static seal. This seal was peeled immediately prior to imaging and the bottom cleaned with compressed air before imaging to prevent interference by dust.


Thus, the simple EIW assay provides a nice quantitative readout of an important worm behavior. While conducting this assay, it is important to control for confounding factors like synchronization, temperature, starvation on plate, contamination, bleach exposure etc. Starvation and contamination both affect the fecundity of worms and therefore can contribute to an artifact in the endpoint. Similarly improper synchronization and exposure to lower or higher temperature can prompt slowed or hastened development leading to incorrect inferences.
Finally, while amenable to high throughput measurement, because so many factors can influence this endpoint, it is essential to conduct this assay on a robust sample size as well as conduct biological replicates to be absolutely certain of the results.
This post would be incomplete if I wrapped up without talking about PERL101 and its performance on these assays. No drumrolls, but consistent with our initial finding, PERL101 exposure at multiple concentrations was found to improve the developmental delay in NPC mutant worms similar to cyclodextrin.