Summary Perlstein Lab PBC (PLab) is Delaware public benefit corporation accelerating drug discovery for the 5,000+ rare genetic diseases. PLab uses a platform of CRISPR-engineered animals (yeast, nematodes, fruit flies and zebrafish) in phenotypic screens to identify orphan drug candidates that reverse disease much faster and cheaper than current approaches. Our proof-of-concept diseases are Niemann-Pick Type C, a lysosomal storage disorder first described nearly a century ago, and NGLY1 Deficiency, a congenital disorder of glycosylation first diagnosed last year.
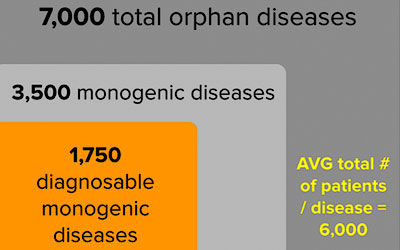
Rationale There are over 7,000 known orphan diseases, which are defined by the Orphan Drug Act of 1983 as diseases affecting 200,000 of fewer patients in the United States. In the US alone there are an estimated 25-30M orphan disease patients. More than 95% of orphan diseases have no FDA approved drugs. Half of all orphan diseases are caused by mutations in a single gene. Of those so-called monogenic diseases, 1,750 are diagnosable, i.e., while the pathophysiology of the disease may be unknown, multi-tissue and complex, the disease-causing gene has been identified. PLab is initially targeting 250 childhood monogenic diseases affecting evolutionarily conserved cellular processes.
Platform overview mutations –> models –> screens –> candidates
Evolutionary conservation Monogenic disease genes are more ancient than the average gene. In other words, most monogenic diseases do not involve uniquely human biology. Therefore, the model organisms depicted below, even a single-cell animal like yeast, are well suited to orphan drug discovery. All animal cells share the same essential set of organelles such as the nucleus, mitochondria, endosomes, lysosomes, etc. This homology extends up to the level of physiology and down to the level of genes: 75% of zebrafish (Danio rerio) genes have a human homolog; 50% of fruit fly (Drosophila melanogaster) genes have a human homolog; 40% of nematode (Caenorhabditis elegans) genes have a human homology; 30% of yeast (Saccharomyces cerevisiae) genes have a human homolog.
Process CRISPR is a new genetic engineering technology that allows us to precisely edit the genome to incorporate any patient mutation. To start, PLab has identified 250 monogenic diseases where the disease-causing gene is conserved in at least one out of four model organisms; out of that 250, approximately 80 diseases can be theoretically modeled in four out of four model organisms, as shown below. Each animal model must be validated to ensure that the human mutation causes the same cellular defects in both patients and models. If so, we design phenotypic screens to discover small molecules that normalize disease phenotypes at both the cellular and organismal levels, e.g., reversal of premature death. Compounds that are ‘hits’ across the platform are likely to target conserved pathways. Such drug candidates, we believe, have greater translational potential than compounds discovered by a traditional in vitro and cell-based drug screening approaches.
Advantages By using a parallel drug screening approach in multiple whole animals that doesn’t rely on a single species or theory of disease, we are much more likely to identify novel chemical entities that fulfill the following criteria:
- Compounds with intrinsically good bioavailability/PK profiles
- Compounds that have low to no toxic off-target effects
- Compounds that require little to no medicinal chemistry in advance of mouse efficacy studies
- Compounds with unexpected, first-in-class mechanisms of action
Platform v1.0 50,000-compound library –> 4-instrument pipeline –> < 1% hit rate
Progress PLab’s lead discovery program for Niemann Pick Type C disease involves nematodes, fruit flies and patient-derived cell lines. With an incidence of 1 in 150,000 live births, NPC has an estimated prevalence of 200-500 patients in the US. At present, there are no FDA approved drugs for the treatment of NPC. 95% of NPC is caused by mutations in the gene NPC1. This gene is responsible for trafficking cholesterol taken up by cells from their environment to lysosomes, the recycling depot of cells. In the absence of NPC1, cholesterol is trapped in lysosomes, where it accumulates. The disorder arises either from an over-abundance of cholesterol or, conversely, a deficiency of cholesterol, which is a precursor for essential downstream end products like hormones, bile acids and cell membrane itself.
In less than a year, we identified 100 fully validated hits. Further characterization in NPC patient fibroblasts revealed that our most promising lead compound, PERL101, which was discovered in our NPC nematode screen, has excellent pharmacokinetic and oral absorption profiles in mice. PERL101 and its analogs are currently being evaluated in NPC mouse toxicity and efficacy studies.
2-3 year goals PLab has identified three additional diseases as prime candidates for our platform. These include NGLY1 Deficiency, Leigh Syndrome (SURF1) and Batten’s disease (CLN3). There are no approved drugs for any of these diseases. Together, these diseases represent a market opportunity of over $400M/year.
5+ year goals At present, it takes nearly 10 years and over $1B dollars to bring a drug to market. Because the underlying disease biology is unknown, orphan diseases are further plagued by poor development timelines. Since 2010, several regulatory policies have been instituted to incentivize the commercialization of orphan products, including pathways for accelerated approval and genetically stratified “small n” clinical trials. There is a high probability of sowing the seeds of future failure in the first five years of the R&D process. PLab is poised to redefine the discovery-to-IND vertical. By building precise genome-edited models and undertaking a parallel, multi-model approach, we will discover transformative orphan drug candidates that are less prone to late-stage clinical failure.